WHO WE ARE
Originally set up as part of GSK’s Global Health Programmes, Positive Action was created in 1992 as the first pharmaceutical company community grant-giving programme to support communities affected by HIV and AIDS.
In 2023, Positive Action has continued working towards its goal of ending AIDS by 2030 by providing funding to community-led and community-based organisations around the world through strategic partner collaboration. Embodying ViiV Healthcare’s mission of leaving no person living with and affected by HIV behind, we have expanded our funding for current and new programmes to increase access to HIV testing, prevention, and treatment services for the communities we serve.
OUR WORK
Positive Action provides funding through two funding mechanisms:
Community Strategic Initiatives
Community Strategic Initiatives (CSI) provide grants to community-led and community-based organisations that are committed to ending AIDS and supporting people living with HIV. CSI funding currently offers funding under two funding streams. Innovator Funding aims to test and pilot new ways of working and provides funding of up to £100,000 over two years. Momentum funding aims to scale-up proven ways of working and funds up to £300,000 over three years. Currently, CSI has over 90 partners implementing projects across Positive Action’s four strategic priorities.
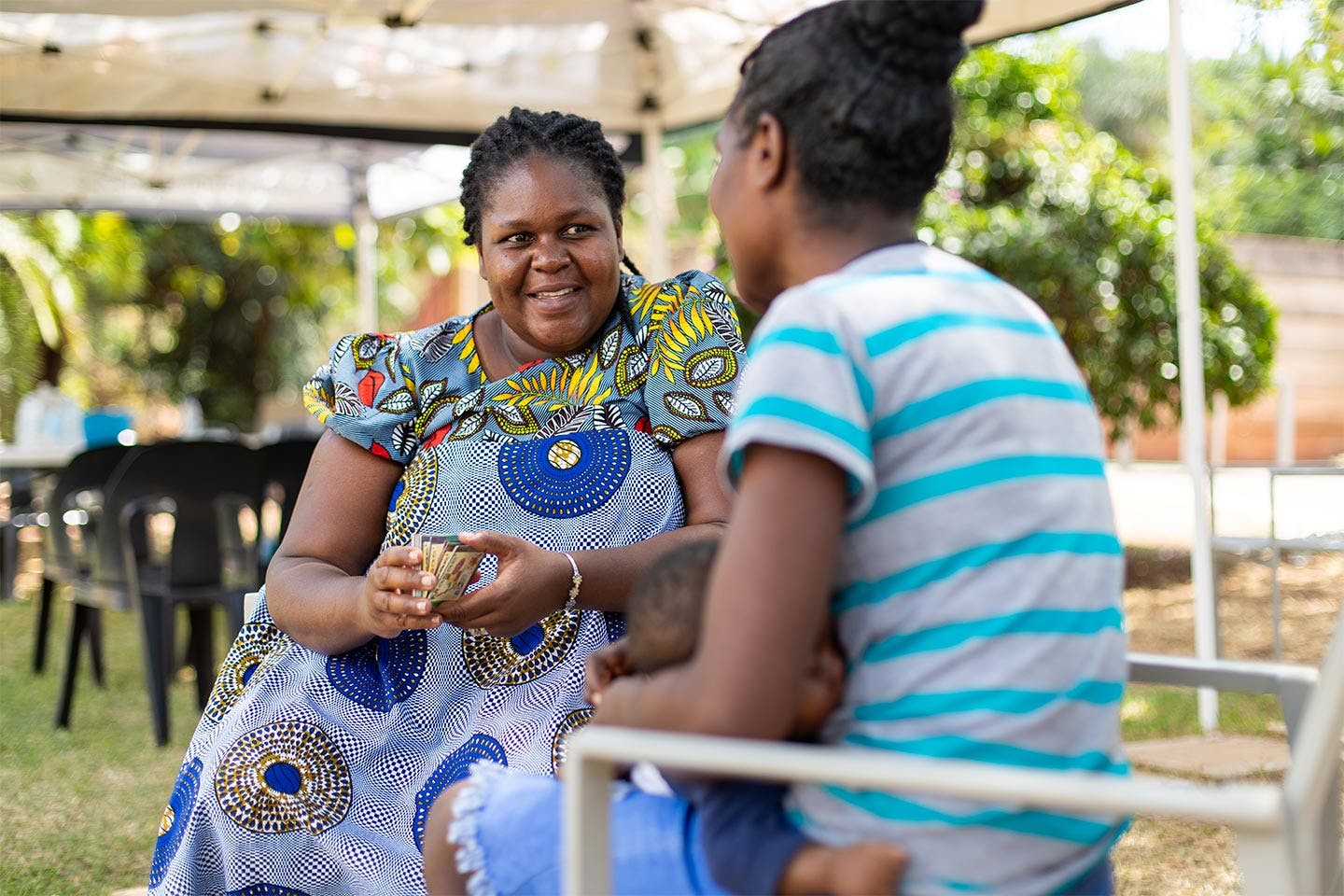
Strategic Partnerships
Strategic partnerships (SP) are informed and shaped by developing, or co-creating shared objectives towards a common outcome, aligned to the Positive Action strategy and broader, high-level HIV-related goals and targets. Partnerships tend to be multi-sectoral and can include other donors and stakeholders, implementing partners, and communities. Strategic partnerships are aligned with Positive Action’s four strategic priorities and are generally not subject to open calls for proposals. Examples of the partnerships include the Paediatric Breakthrough Partnership, the Gender Equality Fund and the HER Voice Fund.
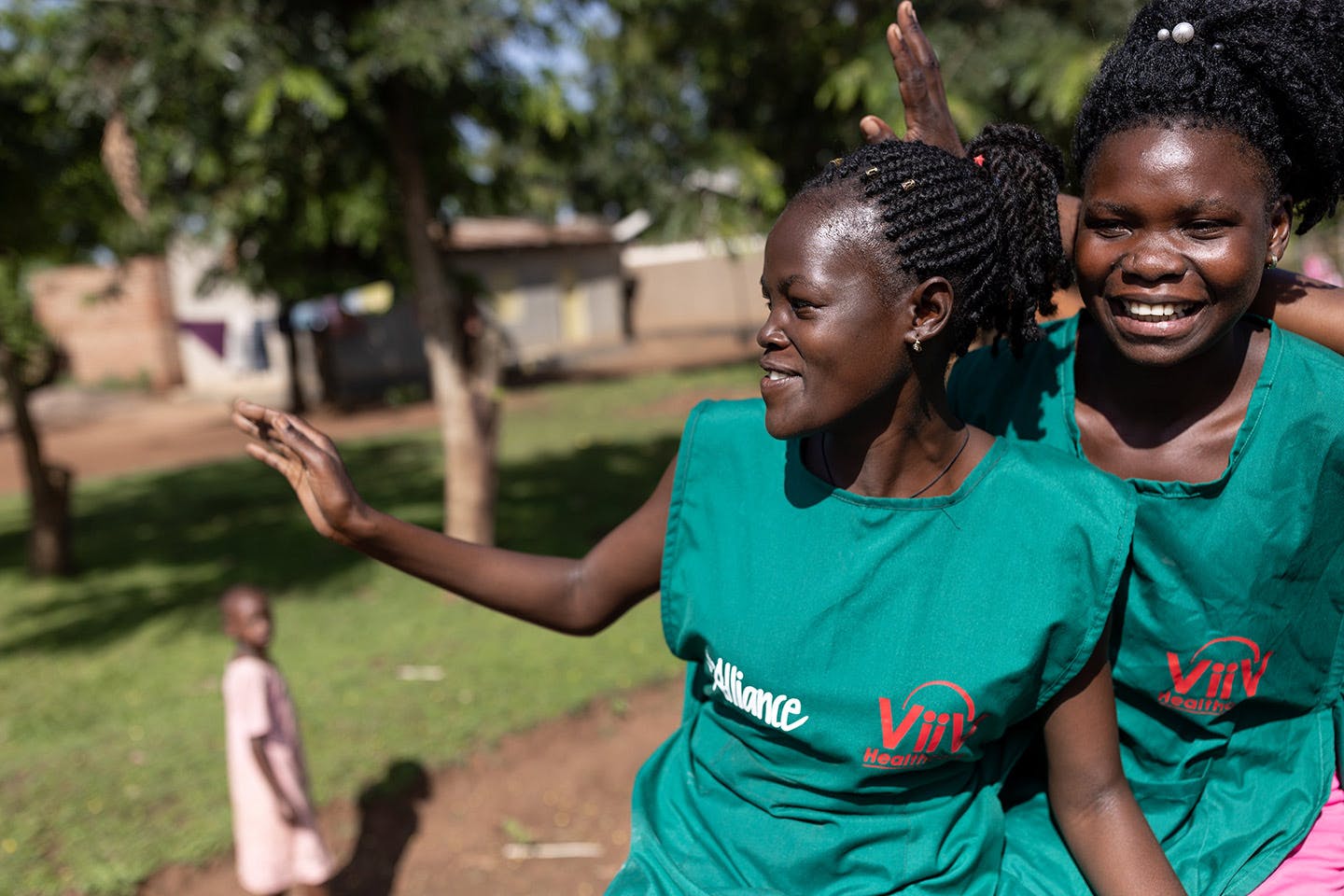
KEY RESULTS
THIS REPORT FEATURES PROJECTS FOCUSED ON
Eliminating HIV-related stigma:
Zvandiri, Zimbabwe

HIV prevention:
Red Somos, Colombia

Harm Reduction:
YouthRISE, Nigeria

Paediatrics:
The Paediatric Breakthrough Partnership, Mozambique, Nigeria and Uganda
WHAT’S NEXT?
As we look to 2024, we are channelling our efforts towards ending paediatric AIDS, reducing HIV-related stigma, increasing awareness and access to harm reduction services for people who use drugs, and implementing HIV prevention strategies. Together with our partners, we will continue to ensure that no one is left behind in our efforts to end the HIV epidemic, by putting people and communities at the forefront of the response.
NP-GBL-HVX-COCO-240017 | April 2024
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard in the Google Play or Apple App store. By reporting side effects, you can help provide more information on the safety of this medicine.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.

